Embark on a journey through AP Chemistry Unit 5 MCQs, where you’ll delve into the fascinating world of acids, bases, and equilibrium. This guide will equip you with the knowledge and strategies to conquer these challenging questions and excel in your AP Chemistry exam.
From understanding the fundamental concepts of acids and bases to mastering complex ion equilibria and redox reactions, this comprehensive guide covers everything you need to know to succeed in AP Chemistry Unit 5.
Unit Overview
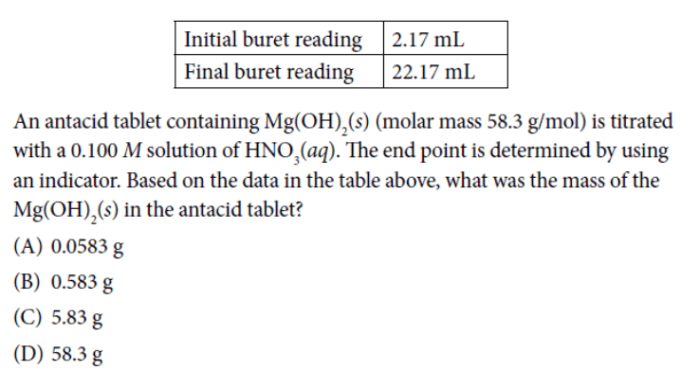
AP Chemistry Unit 5 explores the equilibrium of chemical reactions, a fundamental concept in chemistry that governs the behavior of chemical systems under various conditions. Understanding these principles is crucial for success in the AP Chemistry exam and lays the groundwork for advanced chemistry courses.
Equilibrium involves the dynamic balance between forward and reverse reactions, where the concentrations of reactants and products remain constant over time. This unit delves into the factors that influence equilibrium, such as temperature, pressure, and concentration, and their impact on the equilibrium position.
Equilibrium Constants
Equilibrium constants are mathematical expressions that quantify the extent to which a reaction proceeds towards completion. They provide insights into the relative strengths of reactants and products and can be used to predict the direction of a reaction under different conditions.
Acids and Bases
Acids and bases are two fundamental classes of chemical compounds that play a crucial role in numerous chemical reactions. They exhibit distinct properties and have a wide range of applications in various fields, including chemistry, biology, and industry.
Arrhenius Definition
According to the Arrhenius definition, an acid is a substance that dissociates in water to produce hydrogen ions (H+). Conversely, a base is a substance that dissociates in water to produce hydroxide ions (OH-). This definition is limited to aqueous solutions and does not consider other solvents.
Bronsted-Lowry Definition
The Bronsted-Lowry definition expands the concept of acids and bases beyond aqueous solutions. An acid is defined as a proton (H+) donor, while a base is defined as a proton acceptor. This definition applies to both aqueous and non-aqueous solutions.
Lewis Definition
The Lewis definition is the most general definition of acids and bases. An acid is defined as an electron-pair acceptor, while a base is defined as an electron-pair donor. This definition encompasses all types of chemical reactions, including those that do not involve proton transfer.
Relationship between Acid Strength and Base Strength, Ap chemistry unit 5 mcq
The strength of an acid or base is determined by its ability to donate or accept protons or electrons. Strong acids and bases dissociate completely in water, producing a high concentration of ions. Weak acids and bases dissociate only partially, resulting in a lower concentration of ions.
AP Chemistry Unit 5 MCQs can be tricky, but understanding the underlying concepts is key. Just like the pi kappa phi hand sign is a symbol of unity, grasping the fundamentals of AP Chemistry Unit 5 MCQs will help you achieve success.
Properties of Acids and Bases
Acids and bases exhibit characteristic properties that distinguish them from other substances. These properties include:
- Reactivity: Acids react with bases to form salts and water in a process known as neutralization.
- pH: Acids have a pH below 7, while bases have a pH above 7.
- Conductivity: Acids and bases conduct electricity due to the presence of ions in solution.
Understanding the properties of acids and bases is essential for comprehending a wide range of chemical reactions and processes.
Acid-Base Equilibria: Ap Chemistry Unit 5 Mcq
Acid-base equilibria is a fundamental concept in chemistry that describes the reversible reaction between an acid and a base. When an acid and a base react, they form a conjugate acid-base pair. The equilibrium constant for an acid-base reaction is a measure of the relative strengths of the acid and base.
Equilibrium Constant for an Acid-Base Reaction
The equilibrium constant for an acid-base reaction is expressed as Kaor Kb. Kais the equilibrium constant for the dissociation of an acid, while Kbis the equilibrium constant for the dissociation of a base. The larger the value of Kaor Kb, the stronger the acid or base.
Calculating the pH of a Solution Using the Equilibrium Constant
The pH of a solution can be calculated using the equilibrium constant for the dissociation of water ( Kw). Kwis equal to 1.0 x 10 -14at 25 °C. The pH of a solution is defined as the negative logarithm of the hydrogen ion concentration ([H +]).
pH =
log[H+]
By substituting the equilibrium constant expression for water into the pH equation, we can derive the following equation:
pH = 7
1/2 pKw
where p Kwis the negative logarithm of Kw.
Titrations
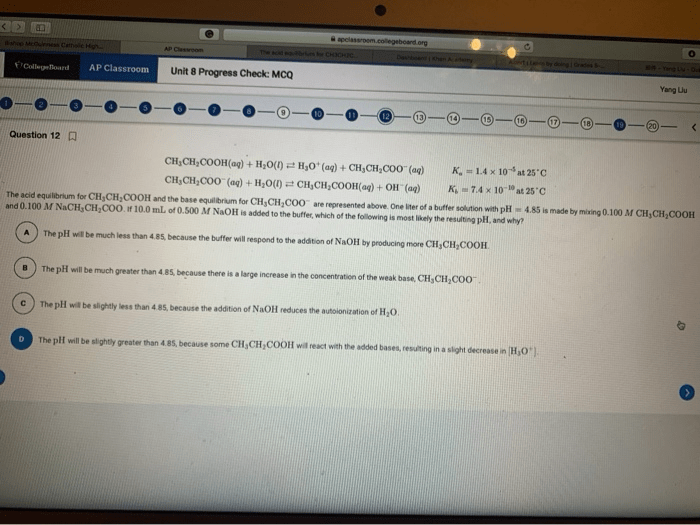
Titrations are a fundamental technique in analytical chemistry used to determine the concentration of an unknown acid or base. They involve the controlled addition of a known concentration of a reagent (titrant) to a solution of the unknown substance (analyte) until a specific endpoint is reached.
The endpoint is typically determined using an indicator, which changes color at or near the equivalence point, the point at which the moles of acid and base are equal. The volume of titrant used to reach the equivalence point can then be used to calculate the concentration of the unknown solution.
Determining the Equivalence Point Using a Titration Curve
A titration curve is a graph that plots the pH of the solution against the volume of titrant added. The equivalence point is the point on the curve where the pH changes most rapidly. This is because at the equivalence point, the moles of acid and base are equal, and the solution is neutral (pH = 7).
Calculating the Concentration of an Unknown Acid or Base
The concentration of an unknown acid or base can be calculated using the following formula:
CaV a= C bV b
where:
- C ais the concentration of the acid
- V ais the volume of acid used
- C bis the concentration of the base
- V bis the volume of base used
This formula can be rearranged to solve for the unknown concentration:
Ca= (C bV b) / V a
Buffers
A buffer solution is a solution that resists changes in pH when a small amount of acid or base is added to it. Buffers work by having a weak acid and its conjugate base or a weak base and its conjugate acid present in the solution.
When a small amount of acid is added, the weak base will react with it to form the weak acid and its conjugate acid, thus preventing a significant change in pH. Similarly, when a small amount of base is added, the weak acid will react with it to form the weak base and its conjugate acid, again preventing a significant change in pH.
The pH of a buffer solution can be calculated using the Henderson-Hasselbalch equation:
pH = pKa + log([A-]/[HA])
where:
- pH is the pH of the buffer solution
- pKa is the dissociation constant of the weak acid
- [A-] is the concentration of the conjugate base
- [HA] is the concentration of the weak acid
Buffers are used in a wide variety of applications in chemistry and biology. They are used to maintain the pH of solutions in chemical reactions, to calibrate pH meters, and to control the pH of biological systems. Buffers are also used in the food industry to control the pH of food products and to prevent spoilage.
Solubility Equilibria
Solubility equilibria refer to the state of equilibrium that exists between a solid solute and its dissolved ions in a solution. When a sparingly soluble ionic compound is added to water, it dissolves to a limited extent, forming a saturated solution.
At equilibrium, the rate of dissolution of the solid is equal to the rate of precipitation of the dissolved ions.
Factors Affecting Solubility
Several factors can influence the solubility of a substance in a solvent. These factors include:
- Temperature:Generally, the solubility of a solid increases with increasing temperature. This is because the higher kinetic energy of the solvent molecules at higher temperatures helps overcome the intermolecular forces holding the solid particles together.
- Pressure:For gases, solubility increases with increasing pressure. This is because the increased pressure forces more gas molecules into solution.
- Nature of the solute and solvent:The solubility of a substance depends on the nature of both the solute and the solvent. Polar solutes dissolve better in polar solvents, while nonpolar solutes dissolve better in nonpolar solvents.
Solubility Product Constant
The solubility product constant (Ksp) is a quantitative measure of the solubility of a sparingly soluble ionic compound. It is defined as the product of the molar concentrations of the constituent ions in a saturated solution. For example, the Ksp for calcium carbonate (CaCO3) is:
Ksp = [Ca2+][CO32-]
The Ksp is a constant for a given compound at a specific temperature. It can be used to calculate the solubility of the compound in water.
Complex Ion Equilibria
Complex ions are formed when a metal ion combines with a ligand, which is a molecule or ion that has at least one atom or ion that can donate a pair of electrons. The resulting complex ion is a new chemical species with different properties than the original metal ion and ligand.
Equilibrium Constant for Complex Ion Formation
The equilibrium constant for a complex ion formation reaction is a measure of the tendency of the reaction to proceed in the forward direction. The equilibrium constant is expressed as the ratio of the concentrations of the products to the concentrations of the reactants at equilibrium.
$$K_\texteq=\frac[\textcomplex ion][\textmetal ion][\textligand]^n$$
where Keqis the equilibrium constant, [complex ion] is the concentration of the complex ion, [metal ion] is the concentration of the metal ion, [ligand] is the concentration of the ligand, and nis the number of ligands that bind to the metal ion.
Calculating the Concentration of a Complex Ion in Solution
The concentration of a complex ion in solution can be calculated using the equilibrium constant and the concentrations of the metal ion and ligand.
$$[\textcomplex ion]=\fracK_\texteq[\textmetal ion][\textligand]^n1+K_\texteq[\textligand]^n$$
Redox Reactions
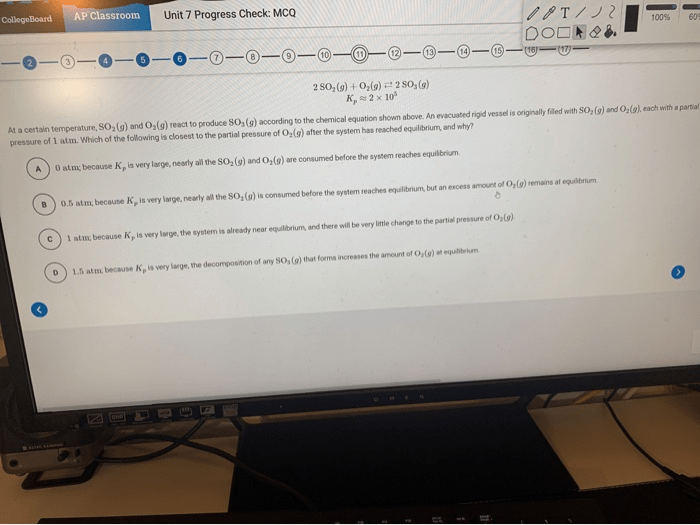
Redox reactions involve the transfer of electrons between atoms or ions, resulting in changes in their oxidation states. Understanding these reactions is crucial in chemistry as they play a vital role in various processes, such as combustion, respiration, and electrochemistry.
Oxidizing and Reducing Agents
In a redox reaction, the oxidizing agent is the substance that accepts electrons and gets reduced, while the reducing agent is the substance that donates electrons and gets oxidized. The oxidizing agent undergoes a reduction half-reaction, gaining electrons, while the reducing agent undergoes an oxidation half-reaction, losing electrons.
Balancing Redox Reactions
Balancing redox reactions can be achieved using the half-reaction method. This method involves separating the reaction into two half-reactions, one for oxidation and one for reduction. Each half-reaction is then balanced in terms of mass and charge. Finally, the two half-reactions are combined to obtain the overall balanced redox reaction.
Electrochemistry
Electrochemistry is the branch of chemistry that deals with the relationship between electrical energy and chemical reactions. It is a fundamental aspect of many important technologies, such as batteries, fuel cells, and solar cells.
The basic principles of electrochemistry can be understood by considering the following simple electrochemical cell:
- A metal electrode is immersed in a solution of its own ions.
- A second metal electrode is immersed in a solution of a different metal ion.
- The two electrodes are connected by a wire.
When the two electrodes are connected, electrons flow from the metal with the lower reduction potential to the metal with the higher reduction potential. This flow of electrons creates an electric current.
The cell potential of a voltaic cell is a measure of the driving force for the electrochemical reaction. The cell potential is equal to the difference in the reduction potentials of the two electrodes.
Calculating the Cell Potential of a Voltaic Cell
The cell potential of a voltaic cell can be calculated using the following equation:
E°cell = E°red(cathode)
E°red(anode)
where:
- E°cell is the cell potential in volts
- E°red(cathode) is the reduction potential of the cathode in volts
- E°red(anode) is the reduction potential of the anode in volts
Q&A
What are the key concepts covered in AP Chemistry Unit 5?
Unit 5 covers acids, bases, acid-base equilibria, titrations, buffers, solubility equilibria, complex ion equilibria, redox reactions, and electrochemistry.
How can I improve my performance on AP Chemistry Unit 5 MCQs?
Practice regularly, review the concepts thoroughly, and seek help from your teacher or a tutor if needed.
What are the most challenging topics in AP Chemistry Unit 5?
Complex ion equilibria and redox reactions are often considered the most challenging topics due to their complexity and the need for a deep understanding of the underlying concepts.